How CDMOs Help Biotech Advance and Navigate Drug Development and Manufacturing Complexities
Nearly 90% of biotech startups fail to bring their discoveries to market, not due to poor science but because they hit operational bottlenecks long before commercialization.
Driven by the growth in biopharmaceuticals, gene therapies, and increasingly complex biologics, biotech companies are facing unprecedented challenges (from regulatory hurdles to supply chain complexity) in scaling their discoveries.
This is where Contract Development and Manufacturing Organizations (CDMOs) step in. A CDMO for biotech becomes a strategic partner, providing both the technical know-how and infrastructure that biotech need without the upfront costs.
What is a CDMO, and Why Do Biotech Need One?
CDMOs for biotech are the one-stop partners capable of supporting everything from process development and scale-up to the full-scale manufacturing of drug substances and products.
This model helps bring novel medicines to market more efficiently and cost-effectively by offering integrated services under one roof.
CDMOs typically support biotech companies by providing services such as:
-
Chemistry and Process Development
-
Clinical trial material production
-
Regulatory support and quality control
-
Packaging and labelling
-
Supply chain and logistics management
These capabilities are especially vital for small and mid-sized biotech firms that may lack the infrastructure to handle such operations in-house. The high capital expenditure is only one barrier.
Regulatory compliance, especially with global regulatory standards combined with Good Manufacturing Practices (GMP), requires highly specialized expertise, infrastructure, and consistent audit-readiness. All of these demands can strain operational bandwidth for early-stage innovators.
By partnering with a CDMO for biotech, companies gain immediate access to cGMP-compliant facilities, experienced staff for regulatory filing and inspections, and equipment suited for both small-batch and commercial-scale production.
Key Benefits of Partnering with a CDMO for Biotech Companies
Biotech companies can achieve greater efficiency and executional support by partnering with a qualified CDMO. Key advantages include:
Accelerated Development and Commercialization
CDMOs have the expertise and capacity to expedite development and production timelines. Their prior experience with similar projects and ready infrastructure helps avoid setup delays, enabling biotech to bring products to patients faster.
Cost Efficiency and Capital Preservation
Building an in-house facility can cost up to $500 million, whereas contracting a CDMO might cost hundreds of thousands per batch for clinical supply.
CDMOs already have the infrastructure and staff in place, so biotech saves on facility investment, maintenance, and personnel overhead. This makes scaling a product far more financially feasible for small companies.
Flexible and Scalable Manufacturing
Manufacturing demand in biotech can be uncertain as a therapy might need to scale up rapidly if trials succeed or scale down if projections change.
CDMOs offer the agility to scale production up or down on short notice without the company being stuck with excess capacity or equipment. This means a biotech can start with small batches for clinical trials and smoothly ramp to commercial volumes if the product is approved, all with the same partner.
CDMOs also mitigate the risk of under- or over-investing in capacity by letting companies pay for just what they need.
Specialized Expertise and Technology
Reputable CDMOs bring deep technical expertise in drug manufacturing and access to advanced technologies that many biotech do not possess in-house.
This includes specialized process know-how, proprietary platform technologies, high-end analytical methods, and equipment for complex modalities (such as cell therapies or high-potency compounds).
For example, Neuland Labs leveraged its deuteration technology to scale a complex API from Phase I to commercial, reducing synthesis steps and cutting raw material costs by one-third.
Regulatory and Quality Compliance
CDMOs specialize in regulatory compliance and have quality systems in place to meet Food and Drug Administration (FDA), European Medicines Agency (EMA), and other international standards. A good CDMO will also assist with documentation for INDs/NDAs and other regulatory submissions.
Letting You Focus on Core Competencies
By outsourcing development and production activities, biotech companies can focus on their core strengths – research, discovery of new therapeutic candidates, and business development.
Drug discovery startups often have lean teams whose primary value is in science and intellectual property.
Handing off the complex manufacturing logistics to a CDMO frees the biotech’s team to concentrate on R&D and pipeline growth. This focused approach can increase the company’s overall productivity and chances of success.
Supply Chain and Risk Management
Established CDMOs have diversified supplier networks and can maintain buffer inventories of key raw materials. They operate multiple manufacturing sites, allowing for contingency plans if one site is interrupted.
During the COVID-19 pandemic, for example, companies working with CDMOs were better positioned to navigate supply disruptions since CDMOs could leverage their scale and supplier relationships to secure needed materials. This level of preparedness and risk mitigation is hard for a single biotech to develop on its own.
Choosing a CDMO vs. In-House Manufacturing vs. Strategic Pharma Partnerships for Your Biotech
Taken together, the above benefits explain why many biotech firms view CDMO partnerships as a smart strategic move. However, as discussed next, it's also important to consider how this model compares to other approaches.
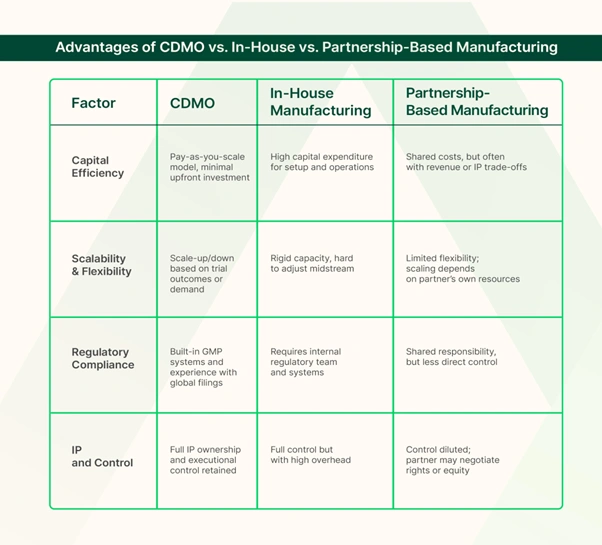
In-House Manufacturing vs CDMO
With in-house manufacturing, your company has direct oversight of production, can prioritize its product, and can protect intellectual property.
In-house production can also potentially yield higher long-term margins per unit (since there are no outsourcing fees) and may make sense for companies with large, stable demand for an approved product.
However, the upfront and ongoing costs are enormous: companies must spend millions on facility construction or leasing, purchase specialized equipment, and recruit and train a full manufacturing workforce.
This ties up capital that could otherwise fund R&D or clinical trials. It also takes significant time to design, build, validate, and get a facility approved by regulators—often several years before the first product is launched.
In contrast, a CDMO can start producing much sooner and adjust volumes as needed without the biotech bearing those fixed costs.
Essentially, in-house manufacturing offers control but demands heavy investment and comes with steep learning curves, whereas CDMOs provide a turnkey solution that trades some margin for speed, savings, and expertise.
Strategic Partnerships vs CDMO
A biotech might license its product to a big pharmaceutical company or form a joint venture with that partner in exchange for scaling up production.
This approach can bring benefits like using the partner’s existing manufacturing facilities and experienced staff without the biotech shouldering all costs. If a reputable pharmaceutical company is involved, it can also validate the biotech’s technology in the eyes of investors.
However, the larger partner may demand significant rights to the product or a share of revenue.
The biotech also becomes dependent on the partner’s priorities and schedule – if the partner has multiple programs, the biotech’s therapy might not always be the top focus.
In contrast, a CDMO is a contracted service provider, so the biotech retains full ownership. The CDMO has a clear contractual obligation to deliver on time and meet quality specs, whereas with a partner company, the lines of accountability can blur.
Additionally, big pharma partnerships can take a long time to negotiate and often require favorable clinical data upfront, whereas engaging a CDMO can happen earlier in development to accelerate progress independently.
Choosing the Right CDMO for Your Biotech
The right CDMO brings scientific depth, regulatory strength, and flexible capacity, empowering small teams to execute like large-scale players.
By offering scalability, expertise, cost savings, and speed, CDMOs enable biotech firms to focus on innovation and strategy while trusting the production process to specialists.
With over 17 years of experience, Neuland Labs has supported the development and scale-up of complex new chemical entity APIs, including deuterated and niche specialty molecules. Its scientific and regulatory expertise has made it a trusted partner to 500+ biotech and pharma companies worldwide.
When you're ready to take your molecule further, contact Neuland Labs for the experience, agility, and scientific depth to help you achieve your goals.
FAQs
|
|
|
|