Your Guide to CDMO Services: What Leading Pharma Teams Rely On
Time is the new currency in pharma. With tightening pipelines, patent cliffs, and market pressure, outsourcing is both tactical and transformational. CDMO services are becoming the operational backbone of modern pharma.
A full-service CDMO offers end-to-end support, providing integrated services that streamline the journey from lab to market. By leveraging CDMO services, pharmaceutical firms gain access to specialized expertise without the substantial investment required to build capabilities in-house.
The result is a transformative effect on development speed, scalability, and regulatory compliance, all of which are critical factors in a successful pharmaceutical journey.
4 Key CDMO Services
Full-service CDMOs offer a broad range of solutions covering every stage of drug development and manufacturing. Key offerings typically include:
1. Drug Development Services
Process R&D and Route Scouting
CDMOs help identify efficient synthetic routes, ensuring processes that are viable for scale-up and commercial manufacturing. This early intervention accelerates lead optimization and reduces development risks.
Stability Testing
To support regulatory submissions and commercial readiness, CDMOs conduct both real-time and accelerated stability studies. These define critical shelf-life and storage parameters and help de-risk the formulation phase.
Analytical Method Development
Analytical methods are developed, validated, and transferred across stages to ensure consistent product quality. This includes impurity profiling, assay methods, and dissolution testing to meet regulatory expectations.
Clinical Trial Material Production
CDMOs support pre-clinical and post-clinical trials by producing flexible batch sizes of drug substance or drug product tailored to each phase of the trial. They manage timeline-sensitive production and enable rapid iteration between trial phases.
2. cGMP Manufacturing
GMP-Compliant Production
CDMOs manufacture APIs, intermediates, and drug products at varying scales under current Good Manufacturing Practices (cGMP), ensuring safety, consistency, and traceability from lab to market.
Global Regulatory Standards
Facilities are designed and audited to comply with major health authorities worldwide, including the FDA, EMA, and PMDA. This global compliance enables seamless international distribution.
Process Validation & Batch Release
As part of commercial drug manufacturing, full-service CDMOs manage full-scale process validation, scale-up, and rigorous QA oversight. Each batch is tested and documented to meet regulatory and quality requirements.
3. Regulatory Affairs & Documentation
Regulatory Strategy Support
CDMOs offer strategic guidance for regulatory filings, including region-specific documentation and ICH-compliant development planning, enabling clients to navigate complex approval landscapes.
Dossier Preparation
In-house regulatory teams support the preparation and submission of INDs, IMPDs, DMFs, and CTD modules, ensuring alignment with global regulatory requirements and submission formats.
Audit & Inspection Readiness
CDMO services include quality audits and inspections, maintaining full data traceability, managing documentation workflows, and ensuring that ongoing cGMP certifications are inspection-ready at all times.
4. Supply Chain & Logistics
Raw Material Sourcing
To mitigate supply risks, CDMOs qualify multiple vendors and source regulated raw materials globally. Supplier networks are optimized for both quality and availability.
Inventory Management
Lean inventory models are deployed for better cost control, with real-time visibility systems to ensure timely procurement, production scheduling, and inventory rotation.
Packaging & Distribution
CDMOs manage global logistics, including labeling, specialized packaging (e.g., for peptides or high-potency APIs), and cold-chain distribution, ensuring the integrity and compliance of commercial pharmaceutical shipments.
How Full-Service CDMO Offerings Solve Pharma Pain Points
Pharmaceutical companies face intense pressure to deliver new therapies faster and at lower cost. Full-service CDMOs directly tackle these pain points by providing holistic solutions:
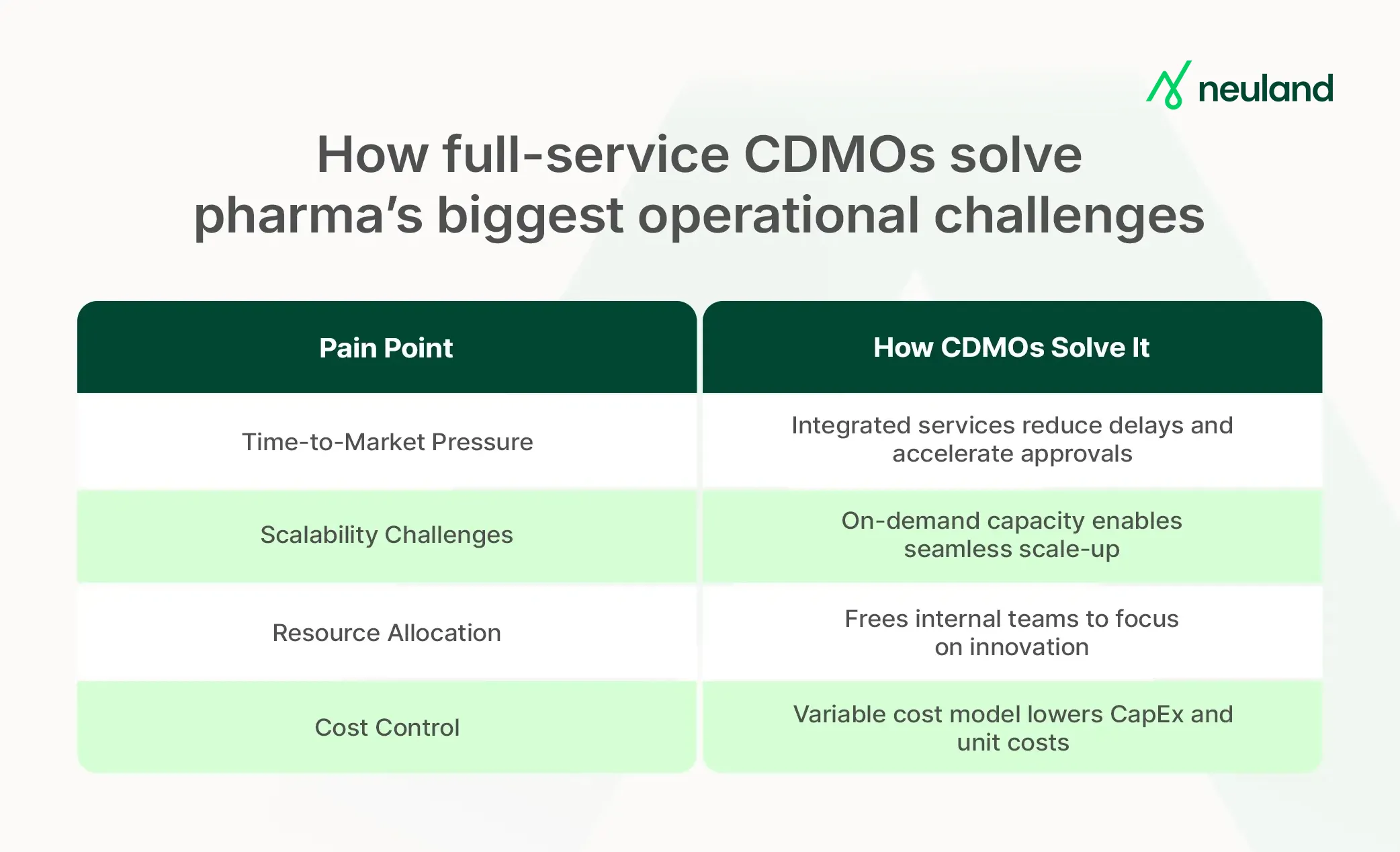
Time-to-Market Pressure
Bringing a new drug from concept to approval typically takes 10 to 15 years and billions of dollars. Every month saved in the process cycle is invaluable.
CDMOs help compress timelines by integrating development and production steps, avoiding handoff delays, and quickly scaling promising candidates. This speed can be decisive in beating competitors to market and extending patent exclusivity.
Scalability Challenges
Scaling up from laboratory bench to commercial volumes is fraught with risk. CDMOs mitigate this by offering flexible capacity and infrastructure on-demand.
Instead of investing in new facilities, pharma companies can tap into a CDMO’s established plants and equipment that are designed for quick changeovers and multiple production scales.
A full-service CDMO, such as Neuland Labs, seamlessly transitions a process from grams to kilograms to tons, using experience from numerous tech transfers to avoid scale-related pitfalls.
Resource Allocation & Cost Control
CDMO pharma services help avoid heavy capex and fixed costs, instead operating on a variable cost model. Companies save the expense of constructing facilities and purchasing equipment for each new project.
Moreover, CDMOs achieve economies of scale by handling projects from many clients, driving down per-unit costs for supplies and production. They also help conserve internal resources, allowing the company’s own scientists to focus on core R&D while the CDMO handles the enabling tasks.
Additionally, by accelerating timelines and averting quality issues, CDMO partnerships reduce the indirect costs of delays and failures.
Neuland Labs: Strategic Advantage of Specialized CDMO Services
While many CDMOs offer broad services, partnering with a specialized CDMO that aligns with your product’s domain can provide a superior strategic fit. Neuland Labs is a global CDMO renowned for its focus on small molecules and peptide APIs. With over 40 years of honing chemistry capabilities, Neuland Labs helps design, develop, and manufacture complex APIs for pharma firms worldwide.
Key strategic advantages of partnering with Neuland Labs include:
- Domain Expertise in Chemistry: Neuland Labs has developed hundreds of processes for diverse chemistries, mastering techniques such as chiral synthesis, hydrogenation, and advanced peptide synthesis. With over 350 scientists in R&D, Neuland can tackle highly complex API projects with confidence. For a pharma partner, that means faster problem-solving and higher yields.
- End-to-End Capability with Minimal Tech Transfer: The company offers a continuum of services from early development (pre-IND) through to full-scale production, providing small-scale clinical trial quantities as well as commercial volumes with minimal tech transfer timelines.
- Proven Track Record of Regulatory and Quality Compliance: The company has successfully completed 25 IND filings and 4 NDA filings, demonstrating extensive experience in meeting global regulatory standards. Its facilities are routinely inspected and approved by major regulators (US FDA, EMA, etc.), and Neuland holds multiple international certifications for quality and safety.
Pharma companies benefit from the convenience of an integrated service provider and a valid extension of their team, offering deep scientific expertise in the relevant domain. Contact us today to know more.
FAQs
|
|
|
|