CDMO vs. CMO: Understanding the Outsourcing Models That Drive Drug Development
Advancing a drug from concept to market is a complex journey with many moving parts.
Biotech and pharma companies often rely on external partners to handle development and manufacturing tasks as their pipelines progress.
Two key types of outsourcing partners in this space are CMOs (Contract Manufacturing Organizations) and CDMOs (Contract Development and Manufacturing Organizations).
Both provide specialized services, but their scopes and strategic value differ in important ways. Choosing between a CDMO vs. CMO is a critical decision that can impact your project’s speed to market, cost-efficiency, and quality.
Let’s understand the differences between CDMO and CMO in a business-focused context and how each model can affect your pipeline drug development strategy.
What is a CMO (Contract Manufacturing Organization)?
CMOs typically engage after a drug’s development is largely complete. Their core role is to manufacture clinical or commercial batches of medicines efficiently and in compliance with regulations.
- Core services: Their primary value proposition is providing state-of-the-art production equipment and expert staff so that sponsors do not need to invest in building their own factories.
- Regulatory compliance: They ensure all production meets current Good Manufacturing Practice (cGMP) requirements set by agencies like the FDA and EMA. They help sponsors stay compliant with these strict standards, which in turn helps avoid regulatory roadblocks on the path to drug approval.
- Industry adoption: CMOs have become an integral part of the pharma supply chain. Whether it’s a big pharma company needing extra production capacity or a virtual biotech without any factories, CMOs provide a means to produce drug candidates at scale with high quality and efficiency.
What is a CDMO (Contract Development and Manufacturing Organization)?
A CDMO goes a step further by offering end-to-end services under one roof. Unlike traditional CMOs, CDMOs integrate research, development, and manufacturing capabilities.
A one-stop solution that can assist a company from the early development stages (such as process development) through clinical trial material production and all the way to commercial manufacturing.
- Core services: CDMOs provide a fully integrated continuum of solutions for drug development and production. This can include process development, analytical method development, scale-up optimization, clinical batch manufacturing, stability testing, regulatory support, and eventual commercial API or drug product manufacturing.
- Integrated model: The integration of development and manufacturing means fewer hand-offs between organizations, which helps simplify workflows and retain knowledge continuity throughout the project. For example, technology transfer between process development and production, often a challenging step, is internalized within a CDMO’s operations, making it far more efficient.
- Strategic value: CDMOs are often seen as long-term partners in a drug’s lifecycle rather than just service providers for one segment. They bring broad expertise that small biotech firms may lack in-house (for instance, process chemists and scale-up engineers all on the same team). This comprehensive support can be especially valuable for complex projects or for resource-constrained companies.
Impact on Pipeline Development: Speed, Cost, and Quality
Outsourcing is a way to accelerate your pipeline effectively. How do CDMOs vs. CMOs compare in speed, cost, and quality? Here’s a strategic overview.
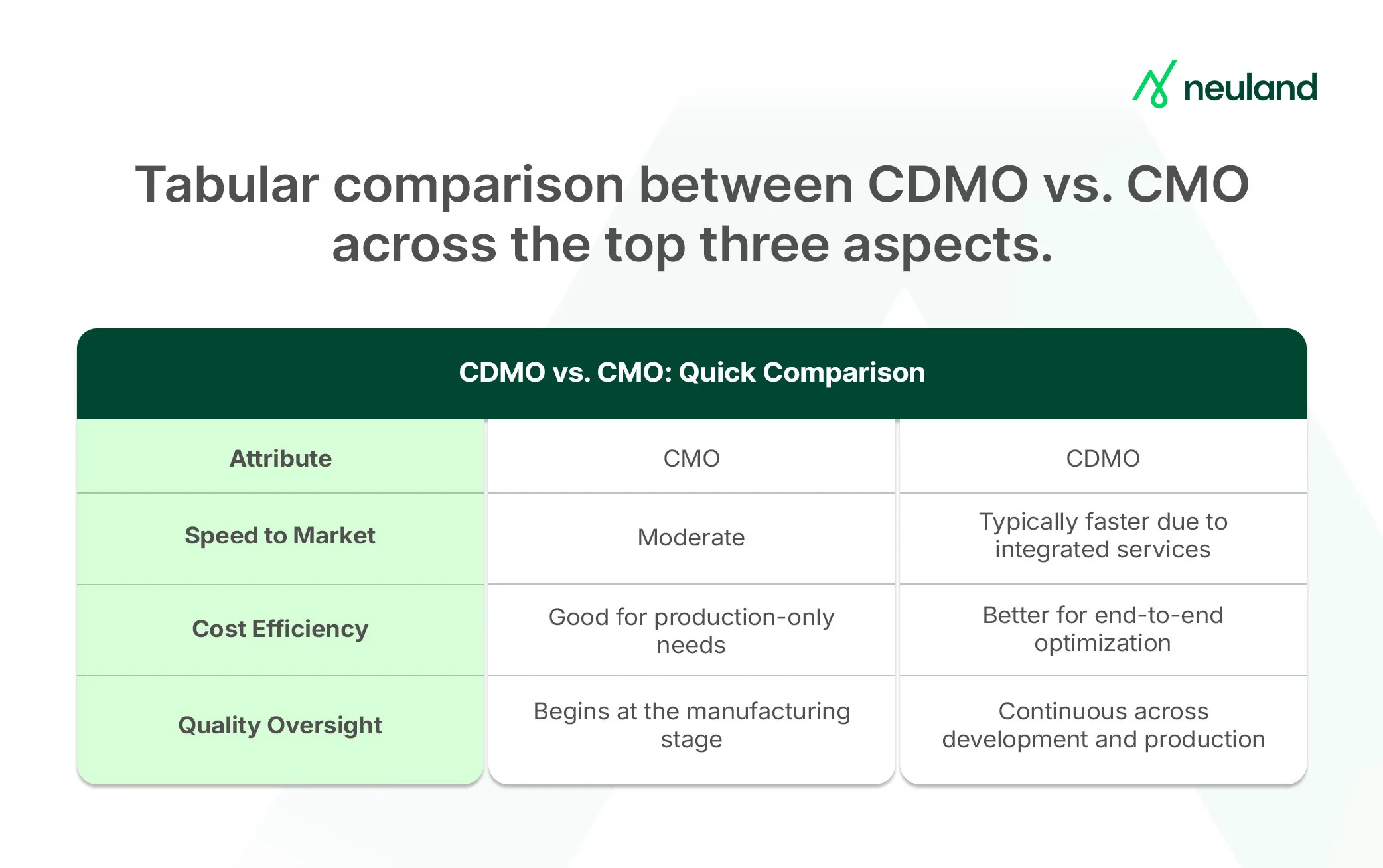
Speed to Market
Handing off development work, such as formulation or process optimization, to a separate CMO often introduces delays. Tech transfer between teams can require repeat experiments, calibration, and problem-solving, slowing progress. This sequential workflow (develop first, then manufacture) makes each hand-off a potential bottleneck.
An end-to-end CDMO streamlines the process.
Once development is complete, manufacturing can begin without pause, within the same team and infrastructure. This eliminates redundant steps and reduces idle time.
If accelerating timelines is critical, a CDMO model offers clear advantages. If internal development is already complete, a well-managed CMO hand-off can still work, provided delays in tech transfer are minimized.
Cost-Efficiency
Both CMOs and CDMOs offer savings, but through different models.
A CMO helps avoid capital investments in facilities, especially valuable for small companies. Their scale allows for bulk purchasing and cost-effective production.
However, using separate vendors for development and manufacturing can lead to duplicated efforts (e.g., repeated studies) and higher project management costs due to coordination gaps.
A CDMO integrates development and manufacturing, reducing duplication and tech transfer costs. Since the same team handles both, there's better process continuity and fewer inefficiencies.
One vendor also means lower administrative overhead and often more flexible, bundled pricing. CDMOs free internal teams for other priorities and shorten time to market, an indirect but significant financial gain.
Quality and Compliance
Top CMOs follow cGMP and have QA systems for manufacturing. However, when development and production are separate, tech transfer becomes a high-risk point.
Miscommunication around process knowledge or critical quality attributes can cause deviations or out-of-spec results. While quality audits and agreements help, oversight must be diligent, especially during hand-off.
CDMOs integrate quality from day one. The same team that develops the process also scales it under a unified QA/QC framework.
This alignment supports Quality by Design principles and reduces surprises during scale-up or regulatory review. Because there’s no break in systems or hand-offs, CDMOs often reduce the risk of quality failures tied to tech transfer.
Both models can deliver compliant products, but CDMO services streamline quality assurance by eliminating hand-offs and unifying development with manufacturing oversight.
CDMO vs. CMO: Choosing the Right Partner for Your Needs
Ask yourself a few strategic questions:
- Do we need only manufacturing, or development support as well?
- How important is it to compress the timeline?
- How complex or novel is our molecule?
- Are we prepared to manage multiple specialized partners, or do we prefer a single point of contact?
The answers to these questions will guide the decision. In case your answer leads you to CDMOs, Neuland Labs is a global CDMO provider that specializes in small-molecule active pharmaceutical ingredients (APIs) and complex chemistry projects.
By leveraging decades of know-how, a partner like Neuland can improve the odds of success, ensuring you can produce affordable, high-purity APIs at scale
FAQs
|
|
|
|