Peptide vs. Protein: Breaking Down 5 Essential Differences for Drug Makers
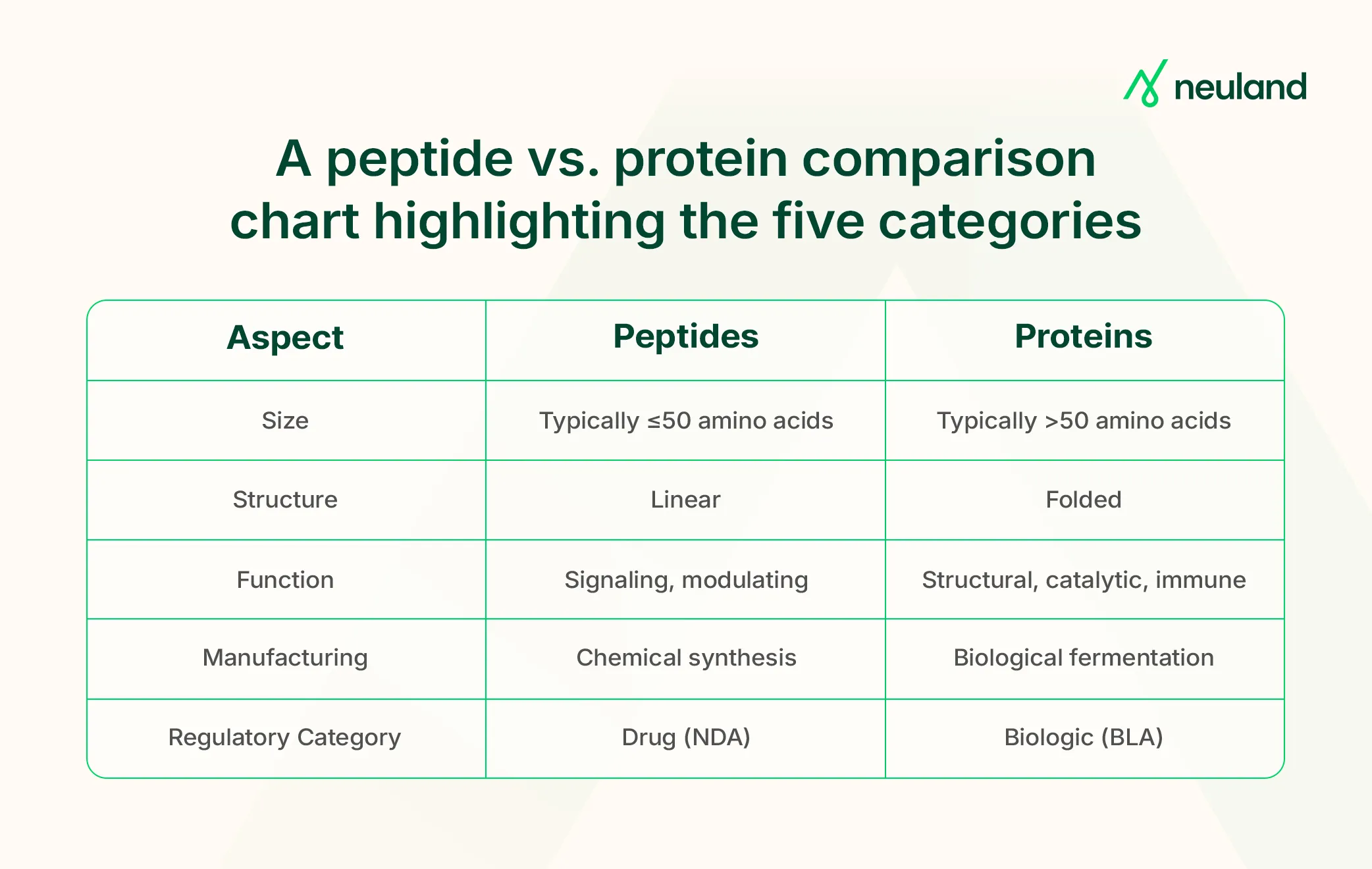
Peptides and proteins are both chains of amino acids held together by peptide bonds, but they differ significantly in size, structure, and how they function and are handled in pharmaceutical development.
For drug makers, the difference between peptides and proteins influences everything from how a molecule is synthesized and stabilized to how it's classified by regulators and delivered to patients.
In this article, we break down five key distinctions that matter most in drug development: structure, biological role, manufacturing methods, stability and delivery, and regulatory pathway.
Whether you're designing a new therapeutic or planning tech transfer, knowing the peptide vs. protein fundamentals can shape your strategy from day one.
Difference #1: Structure & Size
The main distinction lies in size. Peptides are short chains of amino acids, typically 2 to ~50 in length. Proteins, by contrast, are made of 50 or more amino acids, often numbering in the hundreds. Some, like the human protein titin, reach tens of thousands.
This size gap also drives differences in structural complexity. Peptides may form limited secondary structures (like simple folds or helices), but they’re usually too small to maintain stable 3D shapes. Proteins, however, can form elaborate tertiary and quaternary structures, enabling them to function as enzymes, antibodies, or scaffolding materials.
Difference #2: Biological Function
Proteins perform a wide range of essential cellular functions:
- Enzymatic catalysis
- Signal transduction
- Transport and structural support
Thanks to their complex 3D structures, proteins interact with high specificity, making them ideal for therapeutics like:
- Enzyme-replacement therapies
- Monoclonal antibodies
- Cytokines and other biologics
Their targeted action often leads to fewer off-target side effects compared to small molecules.
Peptides, on the other hand, often serve as biological messengers—many hormones and neurotransmitters (e.g., insulin, glucagon, oxytocin) are peptides. In drug development, peptides:
- Bind specific cell receptors
- Block or modulate protein–protein interactions
- Can penetrate cells or deliver payloads intracellularly
This makes them valuable for treating conditions like metabolic disorders, cancer, and infectious diseases, especially when intracellular targeting or receptor specificity is needed.
Though there’s some overlap in (e.g., mini-proteins, enzyme-like peptides), peptides generally modulate biology, while proteins execute complex functions. Both are critical in modern pharma: over 60 peptide drugs are marketed today, with many more in development alongside protein therapeutics.
Difference #3: Manufacturing Approach
A major distinction in the peptide vs. protein discussion lies in how each is made. Peptides are chemically synthesized, while proteins require complex biological expression systems.
Peptide manufacturing relies on chemical methods:
- Solid-phase peptide synthesis (SPPS) is the gold standard. Amino acids are sequentially added on a solid resin, enabling precise chain assembly and high purity
- Solution-phase synthesis is used for shorter peptides or large-scale needs
- Segment condensation joins peptide fragments to build longer chains
These methods avoid live cells, reduce contamination risks, and are generally faster, more economical, and easier to scale than protein biologics. CDMOs like Neuland Labs leverage these methods, with infrastructure tailored to synthetic processes (from short peptides to very long sequences).
Protein manufacturing, by contrast, is biologically driven:
- Recombinant DNA technology is used to express proteins in host cells like E. coli, yeast, or mammalian systems
- It involves developing a stable cell line, culturing in bioreactors, and complex purification to remove host impurities
- Biologic production carries higher costs, more regulatory complexity, and longer development timelines
Peptide manufacturing resembles small-molecule production, while protein manufacturing demands dedicated biologics expertise. In the peptide vs. protein context, this difference significantly impacts speed, scalability, and cost, key considerations for any drug development program.
Difference #4: Stability & Delivery
When it comes to peptide vs. protein, both molecule types pose stability and delivery challenges, but for different reasons.
Chemical and Physical Stability
Proteins are large, fragile, and structurally complex. They can denature or aggregate when exposed to temperature shifts, agitation, pH changes, or light. As a result, many protein drugs require cold chain storage and stabilizers, or are lyophilized for longer shelf life.
Peptides, being smaller and usually lacking a defined tertiary structure, are generally more robust. They often tolerate minor temperature fluctuations better and are commonly lyophilized into stable powders. However, they can still face issues like oxidation, hydrolysis, or aggregation, especially with hydrophobic sequences.
In Vivo Half-Life and Degradation
A major difference between a peptide and a protein is how long each survives in the body.
Peptides are rapidly broken down by enzymes and cleared by the kidneys, giving them short plasma half-lives. Proteins, due to their size, often avoid renal clearance and can circulate longer. Antibodies, for instance, may last days to weeks.
Peptides tend to be less immunogenic, degrading into natural amino acids. But if their sequences are perceived as foreign, they can still trigger immune responses.
Route of administration
Neither peptides nor proteins are suitable for oral delivery without modification. Most require injections. Roughly 75% of peptide drugs are injectable. While some peptides have seen intranasal or transdermal success, large proteins remain injection-only. Advanced drug delivery research is ongoing to improve convenience for both classes.
Difference #5: Regulatory Classification
A key difference between a peptide and a protein lies in how they’re regulated. In the U.S., the FDA classifies peptides (≤40 amino acids) as small-molecule drugs, while proteins (>40 amino acids) are regulated as biologics.
This impacts approval pathways: peptides typically go through a New Drug Application (NDA) and are listed in the Orange Book, while proteins require a Biologics License Application (BLA) and appear in the Purple Book.
The peptide vs. protein classification also determines exclusivity terms, patent challenges (generics vs. biosimilars), and agency review divisions (CDER vs. CBER). For borderline molecules (e.g., 40–50 amino acids), classification can affect development timelines and required studies. In 2020, even insulin (51 amino acids) was reclassified as a biologic.
Ultimately, regulatory agencies draw this line to separate simpler synthetic products from complex biologics that require added oversight.
CDMO Perspective and Neuland’s Peptide Synthesis Expertise
The difference between peptides and proteins has practical implications when partnering with a CDMO. If a pharmaceutical company is developing a peptide therapeutic, it might seek a CDMO with strong peptide synthesis.
Neuland Labs has deep expertise in this arena, spanning solid-phase and solution-phase peptide synthesis, as well as advanced techniques for large and complex peptides, owing to its long history as an API manufacturer.
This means Neuland can produce peptides at scales ranging from R&D grams to multi-kilogram commercial batches, under stringent quality standards, using the most appropriate method for the peptide’s length and complexity.
The ability to incorporate unusual amino acids, create cyclic peptides, perform PEGylation or other modifications, and achieve high purity is critical in peptide CDMO services, and these are areas where specialized peptide manufacturers excel.
Neuland’s expansion of peptide manufacturing capacity, combined with its track record of regulatory compliance and multiple global regulatory approvals over decades, demonstrates the reliability required for peptide APIs. Contact us today to know more.
FAQs
|
|
|
|