What Is Solid Phase Peptide Synthesis? Process, Uses & Benefits
Solid-phase peptide synthesis is a widely used method for assembling peptides step by step on an insoluble solid support.
Introduced by Robert Bruce Merrifield in the 1960s, this method transformed peptide chemistry by replacing the labor-intensive solution-phase process with a more streamlined, efficient approach.
This innovation laid the foundation for today’s automated peptide synthesizers, making solid phase the go-to method for producing high-purity peptides in research and commercial settings.
Step-by-Step Overview of the Solid Phase Peptide Synthesis
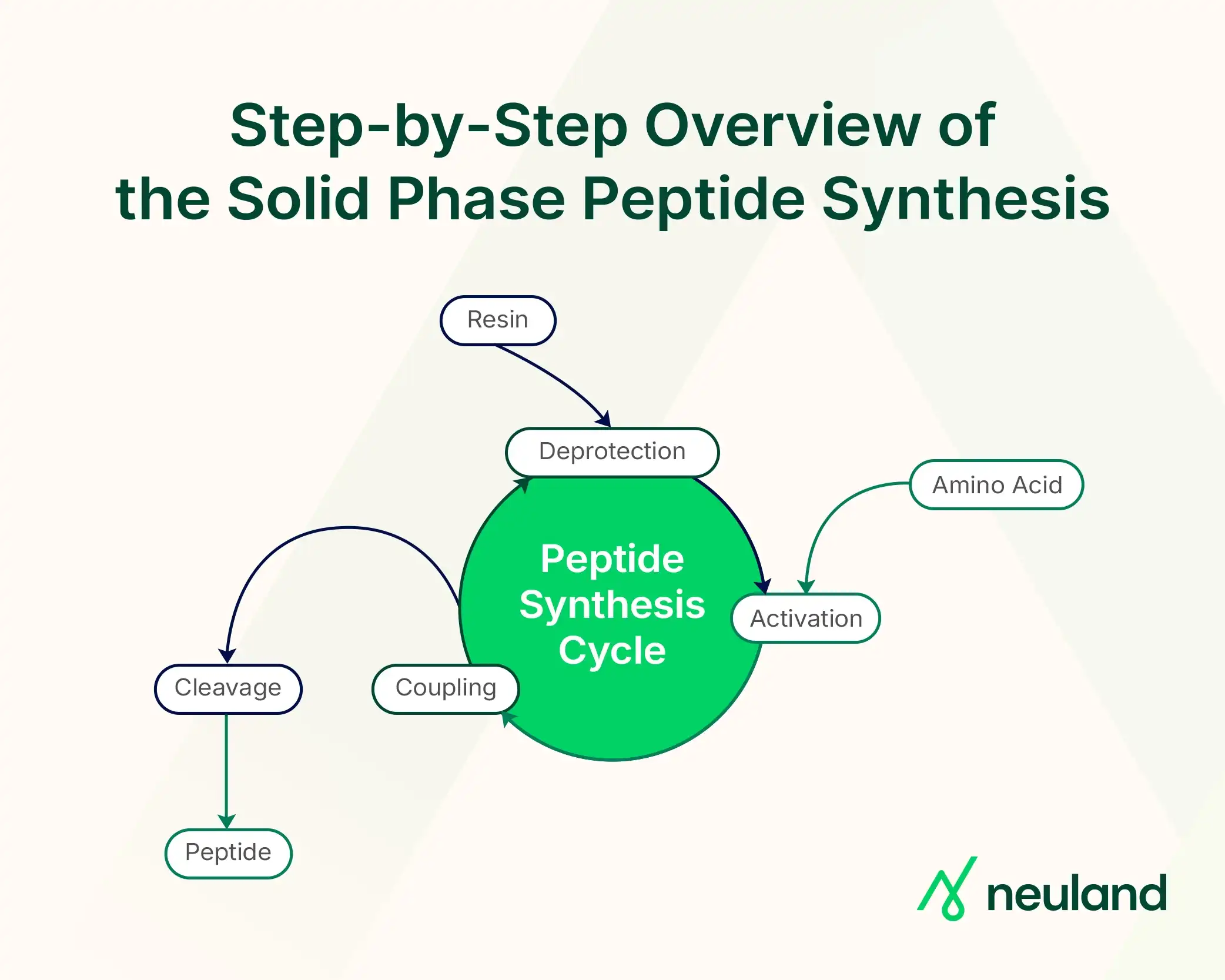
1. Anchoring the First Amino Acid
The process begins by covalently attaching the C-terminal amino acid of the target peptide to a resin bead, i.e., solid support.
The first amino acid is usually pre-loaded on the resin or attached via a linker; its α-amino group is protected (e.g., with an Fmoc group) to prevent self-polymerization.
Anchoring ensures the growing peptide remains insolubilized on the resin throughout synthesis, allowing easy separation of reagents by simple washing
2. Repeating Cycles of Deprotection and Coupling
Each cycle of SPPS involves removing the N-terminal protecting group (deprotection), washing the resin, and then coupling the next amino acid using activating reagents.
Excess reagents drive the reaction to completion, followed by thorough washing. These steps are repeated sequentially, allowing the peptide to elongate one residue at a time.
Multiple wash steps between cycles help remove impurities, ensuring only the correctly assembled peptide remains on the resin.
3. Cleavage from the Resin and Final Deprotection
Once the full peptide sequence is assembled, the completed peptide is cleaved off the resin, and the side-chain protecting groups are removed.
Cleavage is typically achieved with a strong acid like trifluoroacetic acid in the presence of scavengers, which releases the peptide from the solid support and simultaneously strips off acid-labile protecting groups. The peptide is now free in solution.
4. Peptide Purification and Quality Checks
The crude peptide, now in solution, contains the desired product and truncated sequences or other impurities from side reactions.
Purification is usually done by preparative reverse-phase high-performance liquid chromatography, exploiting the peptide’s properties to separate the target product from impurities.
The result is the pure peptide, which can be lyophilized into powder form. Analytical methods such as mass spectrometry and HPLC are used to verify the peptide’s identity and purity.
Solid state peptide synthesis greatly simplifies purification because most of the impurities are washed away during assembly rather than carried through.
Key Advantages of Solid Phase Peptide Synthesis
Efficiency and Speed
Because reactants in solid phase synthesis are used in excess and impurities are washed away each cycle, reactions tend to go to completion quickly. The iterative cycles are easily automated, allowing rapid assembly of complex sequences.
Simplified Purification
The solid support serves as a convenient handle on the peptide. This means fewer chromatographic purifications until the final product, drastically reducing labor.
The result is often a crude peptide that, while impure, is largely free of high-molecular-weight byproducts, making the final HPLC purification more straightforward.
Automation and Scalability
Solid phase peptide synthesis is well-suited to automation using batch peptide synthesizers. Robotic or instrument-controlled systems can perform repetitive deprotection and coupling cycles with precise timing and reagent delivery.
This automation enables scalability from small-scale syntheses up to production scale by simply using larger reaction vessels and more resin.
Flexibility in Sequence and Chemistry
Solid-phase synthesis can accommodate a wide variety of sequences, including difficult or highly functionalized peptides. It is relatively easy to incorporate non-natural amino acids, labels, or modifications at desired positions, since each residue is added independently.
Additionally, if a sequence needs a change, one can often adjust the synthesis without redesigning an entire route.
Limitations of Solid Phase Synthesis and How to Navigate Them
Peptide Length Constraints
In practice, pure peptides longer than ~50 amino acids become difficult to obtain in good yield from a single solid-phase peptide synthesis.
To overcome this, chemists often synthesize large peptides in smaller fragments and then join them (via solution-phase ligation) to reach lengths of 100 amino acids or more.
Chemical Waste and Solvent Use
Solid phase peptide synthesis often requires large volumes of organic solvents for coupling, deprotection, and washing steps. The typical use of 5–10 equivalents of amino acids and coupling reagents per step, plus multiple wash cycles, means significant chemical waste is generated.
This lack of an atom economy is an environmental and cost consideration. Proper waste handling and solvent recycling are needed to mitigate the ecological footprint of the process, especially in large-scale operations.
Solid Phase Synthesis Application in Pharmaceutical and Biotechnology Companies
Therapeutic Peptides
Over 100 peptide therapeutics have gained regulatory approvals across the US, EU, and other regions, and hundreds more are in clinical trials.
Solid-phase peptide synthesis enables the supply of these drugs by providing a reliable means to manufacture peptides under GMP conditions. Many modern peptide drugs for diabetes, cancer, rare diseases, etc., are made via solid-phase methods at commercial scale.
Vaccines and Diagnostics
Peptides are used as antigens in vaccine development and as probes in diagnostic assays. Solid-phase methods allow rapid production of these peptide antigens with precise sequences.
For instance, peptide-based vaccines for viruses, or diagnostic peptides in COVID-19 antibody tests, can be synthesized quickly and adjusted as needed.
Research Tools and Biotech Innovations
The process’s efficiency means even academic labs or small biotech's can order peptides of almost any sequence.
The broad availability of solid-phase made peptides has fueled innovation in fields ranging from materials science to nanotechnology.
Diagnostics and Biomarker Detection
Peptides also serve as the basis for many diagnostic tests and biosensors. For instance, specific peptide sequences can be used to capture antibodies or biomarkers in an immunoassay.
The versatility of the solid-phase method allows tailoring these capture peptides to improve test sensitivity and specificity.
Choosing Neuland Labs for Solid Phase Synthesis
Solid phase peptide synthesis has become the go-to method for producing high-purity peptides efficiently and at scale. But realizing its full potential requires a trusted partner with deep technical expertise.
Neuland Labs brings over 40 dedicated peptide scientists, including post-docs, PhDs, and process engineers, and a proven track record as an approved GMP source for peptide NCE building blocks.
The team recently delivered a 41-amino-acid solid-phase peptide for IND filing (Dec 2024), showcasing both capability and speed.
As an end-to-end peptide CDMO, Neuland offers synthesis, purification, analytics, and regulatory support, all under one roof.
Therefore, Neuland Labs invites innovators to collaborate in harnessing that advantage and to serve as a dependable extension of your development and manufacturing team. Let’s connect.
FAQs
|
|
|
|